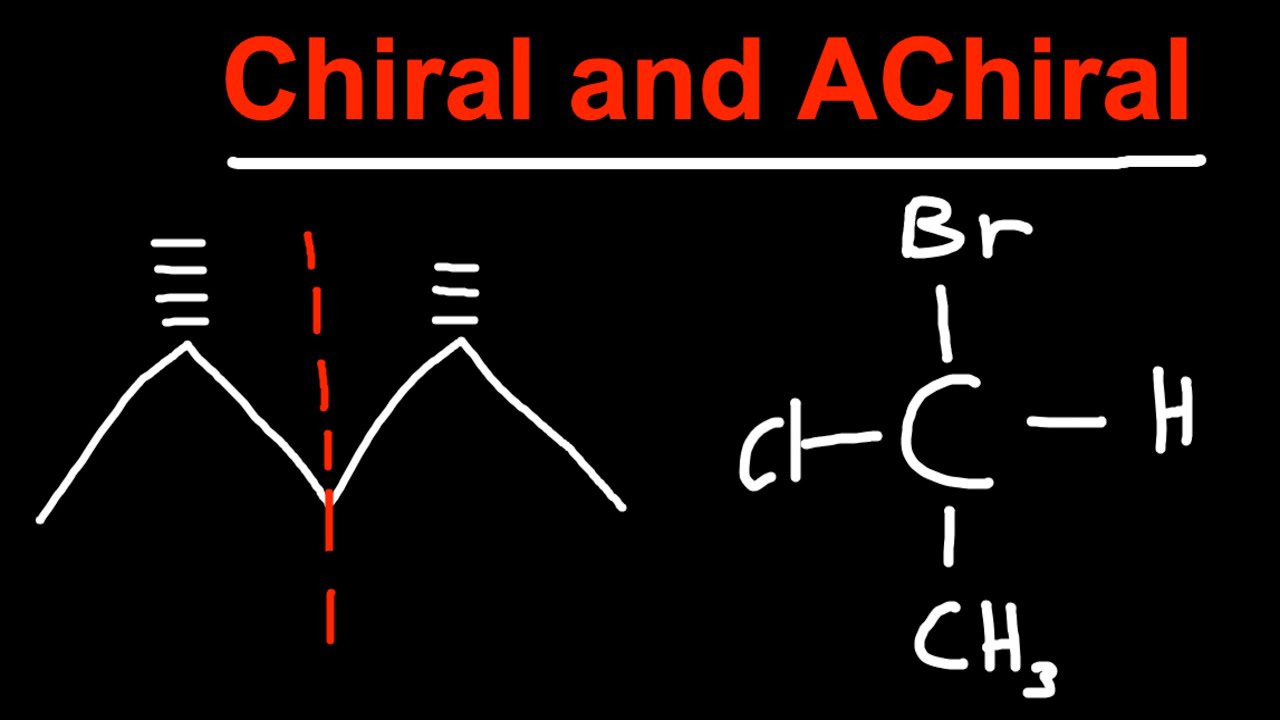
Chiral and Achiral Molecules
The term chiral originates from the Greek word “cheir”, which indicates hand. A chiral molecule or ion is non-superimposable if it cannot be superimposed on its mirror image. This geometric aspect of a molecule is referred as chirality. Chiral molecules lack the center of symmetry and plane of symmetry. Examples include glucose, Naproxen and levopropoxyphene. They exist in two stereoisomers, non-superimposable mirror images of each other and are often referred as enantiomers. Enantiomers are categorized as “right-handed” or “left-handed” according to the absolute configuration. Conversely, Achiral is the opposite of chiral. They are superimposable moles and ion and can be superimposed on its mirror image. Unlike the chiral molecules, achiral molecules possess plane of symmetry or a center of symmetry. A chiral moles possessing stereocenter are identified as Meso molecules. Examples of achiral molecules include (meso)-2,3-dibromobutane and trans-1,2-dichloro-1,2-ethanediol.
What is an Enantiomer?
Enantiomers are pairs of molecules possessing non-superimposable mirror images. They can be recognized by the passing plane polarized light on them. Enantiomers possess similar boiling and melting points.
They have similar melting and boiling points.
What is a racemic mixture?
It is homogenous blend of two enantiomers in a comparative amount known as racemic mixture. A racemic mixture exhibits different composition from enantiomers.